Alternative To Amniocentesis - Amniotic Fluid Testing
Published: 12/14/2015
What is Amniocentesis?
Amniocentesis is a procedure in which amniotic fluid is removed from the uterus for testing or treatment. Amniotic fluid is the fluid that surrounds and protects a baby during pregnancy. This fluid contains fetal cells and various chemicals produced by the baby.
Genetic Amniocentesis
With genetic amniocentesis, a sample of amniotic fluid is tested for certain chromosome/genetic abnormalities - such as Down syndrome and spina bifida (or the open spinal cord in the baby). Arizona Center for Fertility Studies believes that chromosome/genetic amniocentesis should be offered when the test results may have a significant impact on the management of the pregnancy, or the woman's desire to continue the pregnancy.
Maturity Amniocentesis
With maturity amniocentesis, a sample of amniotic fluid is tested to determine whether the baby's lungs are mature enough for birth.
Procedure
Amniocentesis is usually done after the 13-15th week of pregnancy, when the two layers of the fetal membranes, the chorion, and amnion, have fused enough to safely withdraw a sample of amniotic fluid. It is rarely done before that because of safety concerns. Under ultrasound guidance, a sterile needle is passed through the abdominal wall and into the amniotic sac. A small amount of fluid, containing the baby's cells, is removed and sent for genetic/chromosome analysis. The results take about 3+ weeks. This makes the decision to terminate the pregnancy even more difficult since the woman is well into the 2nd trimester, and probably, because she had done a reproductive procedure like IUI or IVF, she has had considerable pregnancy symptoms and probably has watched the baby grow on ultrasound.
Common Indications for Amniocentesis
The following are the most common indications for a woman to consider doing an amniocentesis:
- You had abnormal results from prenatal screening test - AFP (alpha fetal protein) and/or TNL (transnuchal lucency - measurement of the amount of fluid behind the baby's neck).
- You had a chromosomal abnormality (Preimplantation Genetic Diagnosis - PGD/PGS) or neural tube defect in a previous pregnancy.
- You're age 35 or older.
- You have a family history of a specific genetic disorder, or you or your partner is a known carrier of a genetic disorder (Preimplantation Genetic Diagnosis - PGD/PGS).
- You have a suspected uterine infection or Rh incompatibility (where your blood is negative and the baby's and father's blood is positive).
- You are just worried about a chromosome abnormality (Preimplantation Genetic Diagnosis - PGD/PGS) and/or the results may have a significant impact on the management of the pregnancy - or the desire to continue the pregnancy.
Risks Associated with Amniocentesis
Risks associated with amniocentesis are as follows:
- Miscarriage. Early amniocentesis carries a slight risk of miscarriage, often due to rupture of the amniotic sac. The risk of miscarriage is highest when the procedure is done early in pregnancy before the two layers of fetal membranes have been sealed. By the second trimester, however, the risk of miscarriage drops. For years, the risk of miscarriage was generally considered to be 1 in 200. Today, in many centers, the risk is between 1 in 300 and 1 in 500, but only if done by someone with considerable experience and expertise.
- Cramping and vaginal bleeding. Cramping is possible after amniocentesis. Some women experience a small amount of vaginal bleeding, but neither results in pregnancy loss.
- Needle injury. During amniocentesis, the baby may move an arm or leg into the path of the needle. Serious needle injuries are rare.
- Leaking amniotic fluid. Rarely, amniotic fluid leaks through the vagina after amniocentesis. If the leak seals, the pregnancy may proceed normally. Sometimes the leakage can lead to a decrease in the amount of amniotic fluid, that serves as a cushion for the developing fetus. If the leak is severe enough, it can cause skeletal deformities secondary to compression of the baby within the uterus.
- Rh sensitization. Rarely, amniocentesis may cause the baby's blood cells to enter the mother's bloodstream. If the mother is Rh negative but the baby's red blood cells are Rh positive and pass into the maternal blood stream; it makes antibodies against them. This is because the woman's immune system has never seen Rh-positive blood cells. The antibodies are small enough to pass across the placenta and attack the baby's Rh-positive red blood cells and destroy them. If a woman is Rh negative, she should be given RH immunoglobulin (gammaglobulin) after the amniocentesis, so if there is any blood-to-blood transfusion between the mother and the fetus, the gammaglobulin attaches to the baby's Rh positive red blood cells and prevents the mother's immune system from recognizing and attacking them.
- Infection. Although uncommon, amniocentesis may trigger a uterine infection. The resulting infection may trigger premature labor that cannot be stopped or effectively treated with antibiotics.
Arizona Center for Fertility Studies and Amniocentesis
Arizona Center for Fertility Studies recommends that chromosome/genetic amniocentesis should only be offered when the test results may have a significant impact on the management of the pregnancy or the desire to continue the pregnancy. The decision to do an amniocentesis is an extremely important one, especially in reproductive medicine. Because the potential complications of amniocentesis can result in losing a normal pregnancy, and everything that the woman went through to achieve that pregnancy, the risks versus the benefits, needs to be carefully weighed. Arizona Center for Fertility Studies believes, that the decision to terminate a pregnancy secondary to a chromosome abnormality like Downs Syndrome, is an extremely difficult and sometimes emotionally painful one, involving so many emotional, religious, spiritual, psychological, and ethnic aspects. It is a decision that the couple needs to make alone or with family, and the clinic needs to be non-judgmental and support whatever decision they make.
If the AFP and TNL are both normal, there is an 85% chance that the baby is normal and does not have Downs Syndrome. The couple needs to weigh those odds against their risk of having a Downs Syndrome baby and the risk of complications from having the procedure before making their decision.
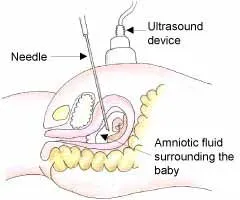
Graphic representation of the amniocentesis procedure done around 14 weeks under ultrasound guidance. A sterile needle is passed through the abdominal wall into the amniotic sac and a small amount of fluid, containing fetal cells, is removed and sent for chromosome/genetic evaluation.
Alternative to Amniocentesis
An alternative to amniocentesis is CVS or chorionic villus sampling. The chorionic villi are tiny finger-shaped growths found in the placenta. The genetic material in chorionic villus cells is the same as that in the baby's cells. During CVS, a sample of the chorionic villus cells is taken for chromosome/genetic evaluation, similar to what is done with the amniotic fluid sample. The main difference between CVS and amniocentesis is that the procedure is generally done late in the first trimester between the 10th to 12th week, and the results are available in 7+ days. This allows the couple to know the health of the baby much sooner and make a decision earlier on whether to continue or end the pregnancy.
The indications and risks of complications are similar to those of amniocentesis and, if the woman decides to have a CVS instead of an amnio, it also has to be done by someone with considerable experience and expertise.
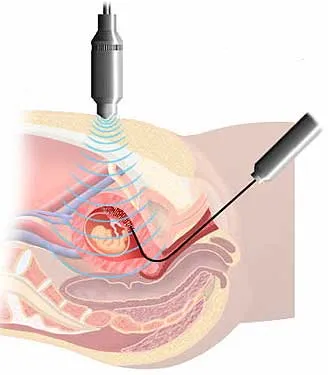
Graphic representation of CVS done under ultrasound guidance. It involves inserting a sterile flexible tube through the vagina and cervix and into the placental tissue removing cells that are genetically/chromosomally similar to the fetus.











